Hydrogen and hydrogen peroxide are two chemical compounds that have similar formulas but different properties and uses. In this article, we will explain what hydrogen and hydrogen peroxide are, how they differ from each other, and some of their common applications.
What is Hydrogen?
Hydrogen is the simplest and most abundant element in the universe. It has the symbol H and the atomic number. It consists of one proton and one electron. Hydrogen can exist as a gas, a liquid, or a solid, depending on the temperature and pressure. Hydrogen gas is colorless, odorless, and highly flammable. It is the main component of water, which has the formula H2O. Hydrogen can also form bonds with other elements, such as carbon, nitrogen, and oxygen, to create various organic and inorganic compounds.
What is Hydrogen Peroxide?
Hydrogen peroxide is a chemical compound that has the formula H2O2. It is similar to water, but it has an extra oxygen atom. Hydrogen peroxide is a pale blue liquid that is slightly more viscous than water. It has a bitter taste and a faint odor. Hydrogen peroxide is a strong oxidizing agent, which means it can accept electrons from other substances and cause them to lose or change their properties. Hydrogen peroxide can also decompose into water and oxygen gas, releasing heat and bubbles. It is used as an oxidizer, bleaching agent, and disinfectant. Hydrogen peroxide can be used in plants as a disinfectant and to promote root growth.
How do Hydrogen and Hydrogen Peroxide Differ?
Hydrogen and hydrogen peroxide differ in several ways, such as:
Structure
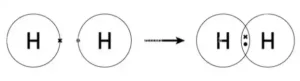
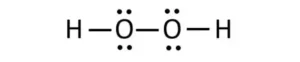
Hydrogen has one oxygen atom per two hydrogen atoms, while hydrogen peroxide has two oxygen atoms per two hydrogen atoms. It has a single bond between the hydrogen atoms, while hydrogen peroxide has a single bond and a double bond between the oxygen atoms. Hydrogen peroxide also has a bent shape, while hydrogen is linear.
Stability
Hydrogen is relatively stable and does not react with many substances unless it is exposed to high temperatures, pressures, or catalysts. It is unstable and can easily decompose into water and oxygen, especially in the presence of light, heat, or impurities. Hydrogen peroxide can also react with many organic and inorganic substances, producing various products and effects.
Uses of Hydrogen and Hydrogen Peroxide
Hydrogen and hydrogen peroxide have different uses, depending on their properties and concentrations.
| Compound | Uses |
|---|---|
| Hydrogen | As fuel for rockets and vehicles Reducing agent in metallurgy As a raw material for ammonia, methanol, and other chemicals |
| Hydrogen peroxide | As a bleaching agent for textiles, paper, and hair It is used as an antiseptic and disinfectant for wounds and surfaces As an oxidizing agent in chemical synthesis and rocket propulsion It is used in plants as a disinfectant and to promote root growth |
Hydrogen is mainly used as a fuel, a raw material, and a reducing agent. For example, hydrogen can power vehicles, produce ammonia and methanol, and remove oxygen from metals.
Hydrogen peroxide is mainly used as a disinfectant, a bleaching agent, and an oxidizing agent. For example, hydrogen peroxide can kill bacteria and fungi, whiten teeth and hair, and synthesize chemicals and explosives.
Conclusion
Hydrogen and hydrogen peroxide are two chemical compounds that have similar formulas but different properties and uses. It is the simplest and most abundant element in the universe, while hydrogen peroxide is a strong oxidizing agent that can decompose into water and oxygen. Hydrogen and hydrogen peroxide have different structures, stability, and uses, depending on their properties and concentrations. Hydrogen and hydrogen peroxide are both important and useful substances, but they also have potential hazards and precautions. Therefore, it is essential to handle them with care and respect.





