Have you ever wondered how we can use hydrogen, the simplest and most abundant element in the universe, to produce electricity? Hydrogen is a clean and renewable source of energy that can power our cars, homes, and devices. But how can we convert hydrogen into electricity? The answer is fuel cells. Let’s learn the hydrogen fuel cell basics.
Fuel cells are devices that use hydrogen and oxygen to create electricity, heat, and water. They are like batteries, but they do not need to be recharged or replaced. They can produce electricity as long as they have fuel and air. Fuel cells are very efficient and environmentally friendly, as they do not produce any harmful emissions or noise.
In this article, we will learn about hydrogen fuel cell basics and types. We also look into their advantages and disadvantages, as well as their applications and challenges, with some examples of how fuel cells are used in real life.
What is a fuel cell?
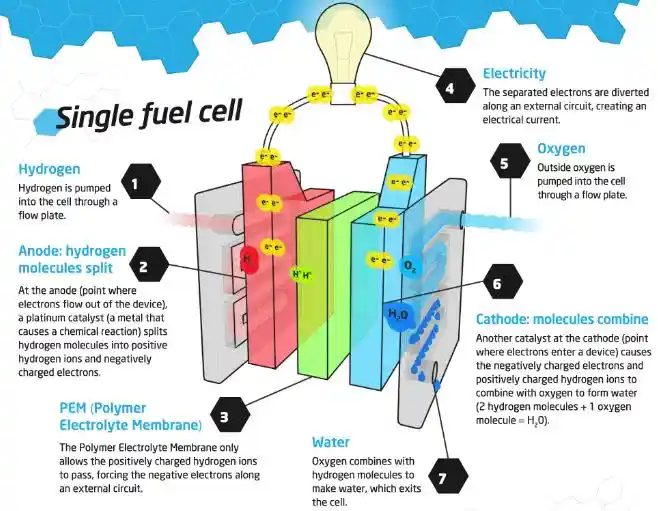
A fuel cell is a device that converts the chemical energy of hydrogen and oxygen into electrical energy. It consists of two electrodes (an anode and a cathode) and an electrolyte. The electrolyte is a substance that allows charged particles to move between the electrodes.
The fuel cell works by splitting hydrogen molecules into protons and electrons at the anode. The electrons flow through an external circuit, creating an electric current. The protons move through the electrolyte to the cathode, where they react with oxygen and the electrons to form water and heat.
The overall reaction of a fuel cell is:
2H2+O2→2H2O+electricity+heat
The diagram below shows how a fuel cell works:
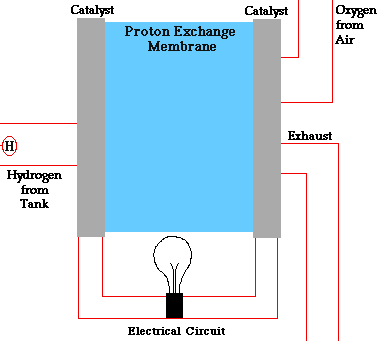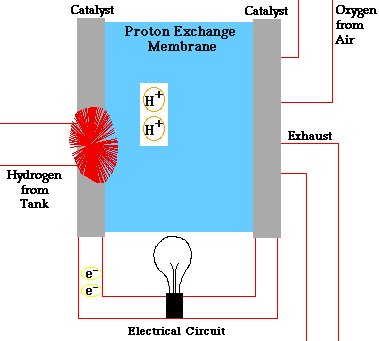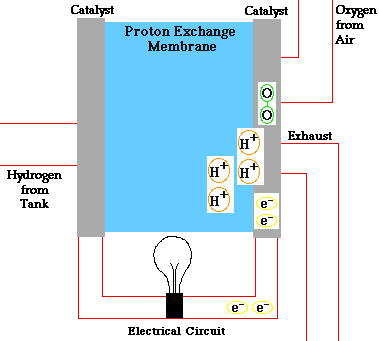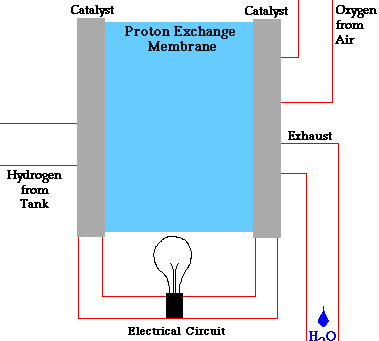
Types of Fuel Cells
There are different types of fuel cells, depending on the electrolyte and the fuel they use. Some of the most common types are:
Polymer electrolyte membrane (PEM) fuel cells
These fuel cells use a thin plastic membrane as the electrolyte and hydrogen as the fuel. They operate at low temperatures (around 80°C) and can quickly adjust to changing power demands. They are suitable for powering vehicles, portable devices, and small-scale stationary applications.
Direct methanol fuel cells (DMFCs)
These fuel cells use a similar membrane as PEM fuel cells, but they use methanol as the fuel. They do not need a fuel reformer, which is a device that converts other fuels into hydrogen. DMFCs operate at low temperatures (around 60°C) and have a high energy density. They are ideal for powering portable devices, such as laptops and mobile phones.
Alkaline fuel cells (AFCs)
These fuel cells use a liquid or a solid alkaline substance as the electrolyte and hydrogen as the fuel. They operate at low to medium temperatures (around 90°C) and have high efficiency. They are used for space applications, such as the Apollo and Space Shuttle missions.
Phosphoric acid fuel cells (PAFCs)
These fuel cells use liquid phosphoric acid as the electrolyte and hydrogen as the fuel. They operate at medium temperatures (around 200°C) and have moderate efficiency. They are used for large-scale stationary applications, such as power plants and hospitals.
Molten carbonate fuel cells (MCFCs)
These fuel cells use a molten mixture of carbonate salts as the electrolyte and hydrogen or natural gas as the fuel. They operate at high temperatures (around 650°C) and have high efficiency. MCFCs can also use the heat they produce to generate more electricity or steam. They are used for industrial and utility applications, such as cogeneration and distributed generation.
Solid oxide fuel cells (SOFCs)
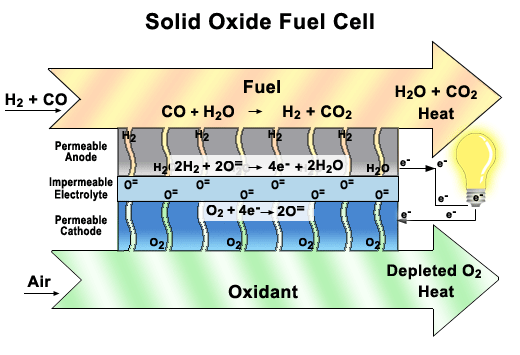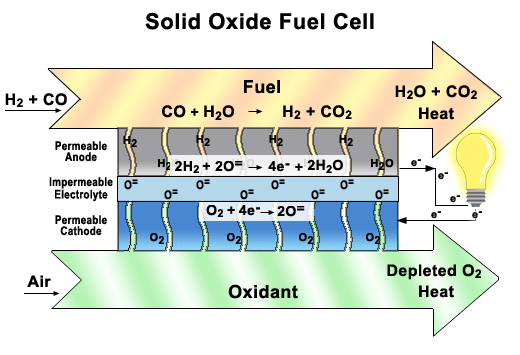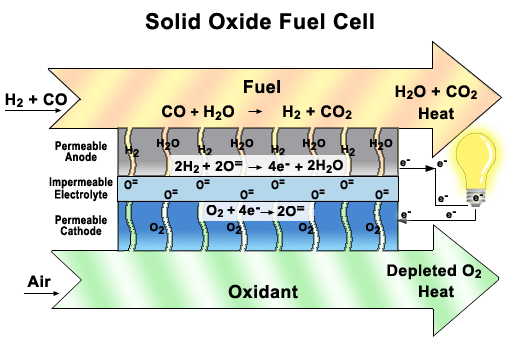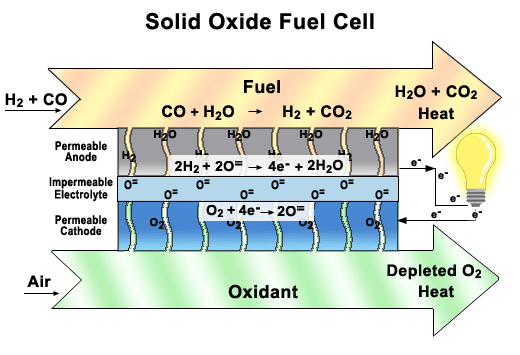
These fuel cells use a solid ceramic material as the electrolyte and hydrogen or natural gas as the fuel. They operate at very high temperatures (around 1000°C) and have very high efficiency. SOFCs can also use the heat they produce to generate more electricity or steam. They are used for large-scale stationary applications, such as power plants and factories.
The table below summarizes the main characteristics of different types of fuel cells:
| Type | Electrolyte | Fuel | Temperature | Efficiency | Application |
|---|---|---|---|---|---|
| PEM | Plastic membrane | Hydrogen | Low (80°C) | High | Vehicles, portable devices, small-scale stationary |
| DMFC | Plastic membrane | Methanol | Low (60°C) | High | Portable devices |
| AFC | Liquid or solid alkaline | Hydrogen | Low to medium (90°C) | High | Space |
| PAFC | Liquid phosphoric acid | Hydrogen | Medium (200°C) | Moderate | Large-scale stationery |
| MCFC | Molten carbonate salts | Hydrogen or natural gas | High (650°C) | High | Industrial and utility |
| SOFC | Solid ceramic | Hydrogen or natural gas | Very high (1000°C) | Very high | Large-scale stationery |
Advantages of Fuel Cells
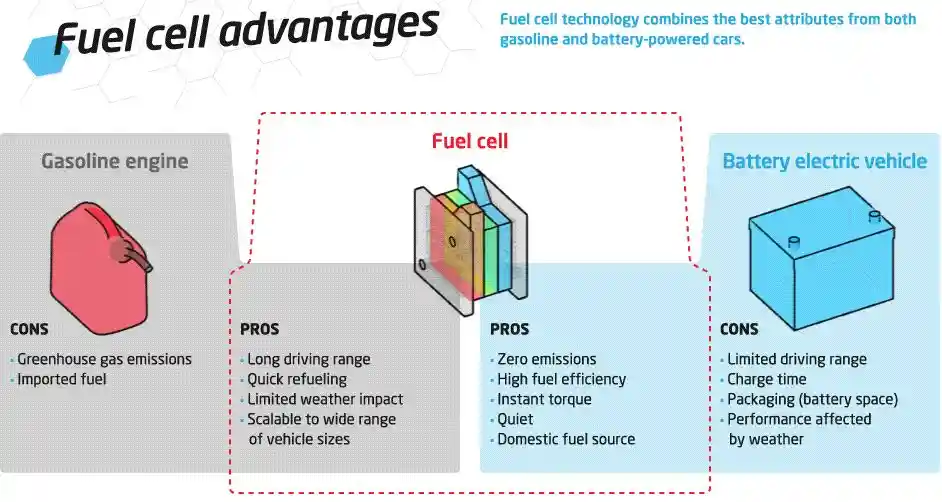
Fuel cells have many advantages over other sources of energy, such as fossil fuels and batteries. Some of the main advantages are:
- Clean: Fuel cells only produce water and heat as by-products and do not emit any greenhouse gases or pollutants. They can help reduce the environmental impact of energy production and consumption.
- Efficient: Fuel cells can convert up to 60% of the fuel’s energy into electricity, and up to 85% if heat is also used. This is much higher than the efficiency of conventional power plants, which is around 35%.
- Reliable: Fuel cells can provide a steady and continuous supply of electricity without any interruptions or fluctuations. They do not depend on the weather, the time of day, or the availability of the grid. They can also operate in harsh conditions, such as high altitudes or extreme temperatures.
- Flexible: Fuel cells can use a variety of fuels, such as hydrogen, natural gas, methanol, ethanol, or biogas. They can also be combined with other renewable sources, such as solar or wind, to create hybrid systems. They can also be scaled up or down to meet different power needs, from watts to megawatts.
Disadvantages of Fuel Cells
However, fuel cells also have some disadvantages and challenges that need to be overcome. Some of the main disadvantages are:
- Costly: Fuel cells are still very expensive to produce and maintain, compared to other sources of energy. They require high-quality materials, such as platinum, and complex components, such as fuel reformers and compressors. They also have a limited lifespan and need to be replaced periodically.
- Safety: Fuel cells involve the use of flammable and explosive fuels, such as hydrogen and methanol, which pose a risk of fire or explosion. They also produce high temperatures and pressures, which require special handling and storage. They also need to comply with strict safety standards and regulations.
- Infrastructure: Fuel cells require a well-developed and accessible infrastructure for the production, distribution, and storage of the fuels they use. This is especially challenging for hydrogen, which is not widely available and has a low energy density. It also requires new pipelines, stations, and tanks, which are costly and time-consuming to build.
Applications of Fuel Cells
Fuel cells have a wide range of applications and potential markets, from transportation to power generation. Some of the current and future applications and examples of fuel cells are:
Transportation
Fuel cells can power vehicles, such as cars, buses, trucks, trains, boats, and planes. They can offer a longer range, faster refueling, and lower noise than batteries. They can also reduce the dependence on oil and the emissions of carbon dioxide and other pollutants.
Examples of Fuel Cells Vehicles
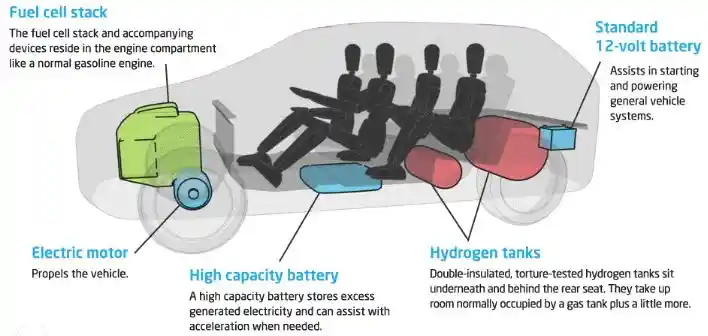
Some examples of fuel cell vehicles are:
- The Toyota Mirai is a hydrogen fuel cell car that can travel up to 500 km on a single tank of hydrogen and emit only water vapor.
- The Hyundai Nexo is another hydrogen fuel cell car that can travel up to 600 km on a single tank of hydrogen and emit only water vapor.
- The Honda Clarity is a hydrogen fuel cell car that can travel up to 589 km on a single tank of hydrogen and emit only water vapor.
- The Ballard FCveloCity is a hydrogen fuel cell bus that can travel up to 350 km on a single tank of hydrogen and emit only water vapor.
- The Alstom Coradia iLint is a hydrogen fuel cell train that can travel up to 1000 km on a single tank of hydrogen and emit only water vapor.
- The ZeroAvia ZA-600 is a hydrogen fuel cell plane that can fly up to 800 km with six passengers and emit only water vapor.
Power generation
Fuel cells can provide electricity and heat for buildings and communities, such as homes, offices, schools, hospitals, and factories. They can offer high efficiency, low noise, and a reduced carbon footprint. They can also operate independently or in conjunction with the grid, providing backup power or peak shaving.
Examples of fuel cell power generation
Some examples of fuel cell power generation are:
- The Bloom Energy Server is a solid oxide fuel cell system that can produce up to 250 kW of electricity and heat from natural gas or biogas.
- The Doosan PureCell is a phosphoric acid fuel cell system that can produce up to 400 kW of electricity and heat from natural gas or biogas.
- The FuelCell Energy SureSource is a molten carbonate fuel cell system that can produce up to 2.8 MW of electricity and heat from natural gas or biogas.
- The Ene-Farm, a polymer electrolyte membrane fuel cell system that can produce up to 1
Portable Devices
Fuel cells can power small and portable devices, such as laptops, mobile phones, cameras, and drones. They can offer longer battery life, a lighter weight, and a lower environmental impact than conventional batteries. They can also be recharged quickly and easily with replaceable fuel cartridges.
Examples of fuel cell portable devices
Some examples of fuel cell portable devices are:
The Intelligent Energy Upp, is a polymer electrolyte membrane fuel cell charger that can provide up to five full charges for a smartphone or a tablet.
The Horizon MiniPak, a direct methanol fuel cell charger that can provide up to 10 hours of power for a smartphone or a camera.
The Intelligent Energy Fuel Cell Drone is a hydrogen fuel cell drone that can fly for up to 2 hours with a 5 kg payload and emit only water vapor.
Military Applications
Fuel cells can provide power and heat for military applications, such as vehicles, bases, communications, and weapons. They can offer a silent operation, a reduced thermal signature, and lower fuel consumption than diesel generators. They can also enhance the security and reliability of the power supply and reduce the logistics and costs of fuel transportation.
Examples of fuel cell military applications
Some examples of fuel cell military applications are:
- The General Motors ZH2 is a hydrogen fuel cell truck that can travel up to 400 km on a single tank of hydrogen and provide 25 kW of exportable power for other devices.
- The Protonex M250-CX is a solid oxide fuel cell system that can provide up to 250 W of power for communications and surveillance equipment.
- The Ultra Electronics Bren-Tronics SOFC Hybrid System is a solid oxide fuel cell system that can provide up to 3 kW of power for remote and forward operating bases.
Space Applications
Fuel cells can provide electricity and water for space applications, such as satellites, rockets, and spacecraft. They can offer more specific energy, a longer duration, and lower maintenance than solar panels or batteries. They can also reduce the weight and volume of the spacecraft and increase mission flexibility and capability.
Examples of fuel cell space applications
Some examples of fuel cell space applications are:
- The Apollo Service Module was a hydrogen-oxygen fuel cell system that provided up to 1.4 kW of power and 0.9 kg of water per hour for the Apollo missions to the moon.
- The Space Shuttle Orbiter was a hydrogen-oxygen fuel cell system that provided up to 12 kW of power and 7 kg of water per hour for the Space Shuttle missions to the low Earth orbit.
- The Orion Multi-Purpose Crew Vehicle is a hydrogen-oxygen fuel cell system that will provide up to 5 kW of power and 3 kg of water per hour for future missions to the moon and beyond.
Conclusion
Fuel cells are a promising technology that can enable a clean and efficient energy transition for various applications and sectors. They can convert hydrogen and oxygen into electricity, heat, and water without producing any harmful emissions or noise. They can also use different types of fuels, such as natural gas, methanol, or biogas, and integrate with other renewable sources, such as solar or wind.
However, fuel cells also face some challenges and barriers that need to be overcome, such as high costs, safety issues, and infrastructure gaps. They also need to compete with other energy technologies, such as batteries, combustion engines, and turbines. Therefore, fuel cells require more research and development, innovation and collaboration, and policy and regulation to achieve their full potential and benefits.




